GFPD is Advocacy!
Beginning in 2017, the GFPD started training our families and professionals to advocate on behalf of legislation that impacts both the peroxisomal disorder community and the rare disease community.
To learn more about the GFPD’s advocacy efforts, email: Ryan@thegfpd.org
What Are We Advocating For?
Interagency Coordinating Committee
The Need: Federal rare disease programs and projects occur across the entire government aimed to address challenges within the rare community such as the diagnostic odyssey, a limited rare disease therapy pipeline, and access to care and treatments.
The Proposed Solution: Interagency coordinating committees have been successful in assessing existing federal initiatives and identifying opportunities for improvements, ensuring one agency initiative can have maximum impact through coordination with other agencies, tracking metrics that can show progress or other areas where resources are needed.
The creation of an interagency coordinating committee will provide a central location for sustained coordination of the federal government’s rare disease initiatives.
Reauthorize the Rare Pediatric Disease Priority Review Vouchers
The purpose of PRV’s is to expedite the development and promotion of drugs in the rare pediatric diseases. Developing drugs for rare pediatric diseases is particularly challenging due to the small populations affected, difficulties associated with conducting clinical trials for children, delays in diagnosis, and more. A majority of PRVs have gone to rare pediatric disease drugs, meaning the program has a large impact on the rare disease community.
It is vital to reauthorize the Priority Review Voucher program, which provides incentives to pharmaceutical companies for developing treatment for pediatric rare diseases. Companies can either use the voucher (or incentive) to accelerate the approval of another drug they are developing, or they can sell it to another pharmaceutical company.
Join the Rare Disease Congressional Caucus
House Co-Chairs: Representative Doris Matsui (CA) and Representative Gus Bilirakis (FL)
Senate Co-Chairs: Senator Roger Wicker (MS) and Senator Amy Klobuchar (MN)
Find out if your members of Congress are involved in the Rare Disease Caucus
Learn more about the Rare Disease Congressional Caucus
- Gives the rare disease community a permanent voice of Captiol Hill
- Holds quarterly Caucus briefings to update Congressional Staff on important policy issues
- We want every member of Congress to not just know about rare disease but to know a rare disease patient in their district
Support the Accelerating Kids’ Access to Care Act
Currently, if a patient needs to see a provider outside of their state the provider must become a part of the patient’s state’s Medicaid program, which is a long-time consuming process.
This bill would change this process by making a new, faster process for doctors to enroll in another state’s Medicaid program by:
- Establishing a federal streamlined screening and enrollment pathway
- Voluntary, meaning providers are not required to do this
- If providers opt to be screened through this pathway, they will be eligible to enroll in other state’s Medicaid programs for a 5-year period, assuming they remain in good standing
- Eligible providers must present the most limited risk of fraud and abuse, be enrolled in Medicare or their home state Medicaid program, and not be excluded from participating in any federal health programs
Rare Disease Community Letter to the United States President
A group of over 200 rare disease communities banded together to write a letter to President Trump to provide him with a set of priority policies to support the rare disease community as he began his term as our nation’s 47th President.
The policy priorities articulated in this letter are informed by the needs of our community and our shared missions of ensuring all Americans affected by one or more of the estimated 10,000 rare diseases can access a timely diagnosis, expert clinical care, and optimal treatment options. We are a community who feels an intense sense of urgency on behalf of the millions of Americans living with rare diseases and their families, a disproportionate percentage of whom are children.
Deafblind Day On the Hill – Cogswell-Macy Act
Cogswell-Macy Act
The Cogswell-Macy Act is intended to improve the effectiveness of special education and related services for children and youth who are Deaf/Hard of hearing, Blind/Visually Impaired and DeafBlind
The Cogswell Macy act consists of three parts:
- Title 1 is focused on Deaf and Hard of Hearing
- Title 2 is focused on blind and visually impaired
- Title 3 is focused on Deafblind our children
- Elements in Title 3 is what is needed for individuals with deafblindness to access their education
The Cogswell-Macy Act was first introduced with Title 3 in 2015, but was sent to committee for review each legislative session and died without ever going through bill markup to let us know why it was not moved forward.
In March 2025, Representative Morgan McGarvey (KY) plans to introduce the Cogswell-Macy Act, along with a new bill, the Helen Keller Education Act.
Take action now by letting your legislators know about these, and once the Cogswell-Macy Act and Helen Keller Education Act are both introduced, ask your representatives to support both.
Since it has not been introduced yet, there is no Bill number to provide. Prior to contacting your representatives, it may be helpful to review the previous list of 2023-2024 Cogswell-Macy Act Co-Sponsors. If they have previously co-sponsored, please thank them when informing them about the upcoming reintroduction of the bill, and about the new one as well.
Deafblind Day On the Hill – Helen Keller Education Act
Helen Keller Education Act
The Helen Keller Education Act will enhance the Individuals with Disabilities Act (IDEA) to meet the complex needs of individuals who are DeafBlind. It is essentially Title III of Cogswell-Macy as its own bill. Elements in Title 3 of the Cogswell-Macy Act are what is needed for individuals with deafblindness to access their education.
In March 2025, Representative Morgan McGarvey (KY) plans to introduce the Helen Keller Education Act, along with the Cogswell-Macy Act.
Take action now by letting your legislators know about this new bill. Once the Cogswell-Macy Act and Helen Keller Education Act are both introduced, ask your representatives to support both.
It is important to note that until it is introduced, there is no Bill number to provide.
Deafblind Day On the Hill – DeafBlind Congressional Caucus
DeafBlind Congressional Caucus
This new Deafblind Congressional Caucus will be dedicated to:
- Highlighting and amplifying the priorities of the deafblind community
- Encouraging the creation, and implementation, of legislation that advances the priorities and elevates the needs of this community
The new DeafBlind Congressional Caucus currently has 6 members and needs 10 to officially be established. The founding members are: *McClain Delaney, Carson, Doggett, McGarvey, Mullin, Norton
Take action now by asking your Representatives to join the Deafblind Congressional Caucus.

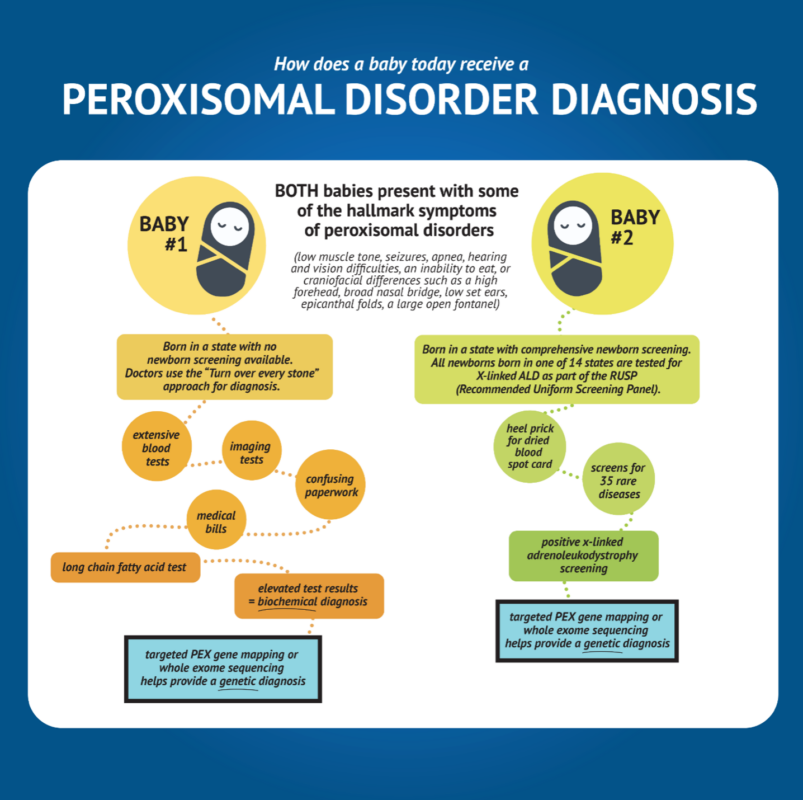

Importance of Newborn Screening for the GFPD Community
Newborn screening is an incredible tool to get families closer to receiving the support they need to give their loved one the best quality of life possible.
Receiving an accurate diagnosis can help eliminate a difficult and expensive diagnostic odyssey.
The earlier GFPD families can learn about peroxisomal disorders, the earlier they can focus on beneficial therapeutic interventions and getting the best treatment available.
In December 2021, the Journal of the American Medical Association Network Open (JAMA Network Open) published a first-of-its-kind study, Expert Evaluation of Strategies to Modernize Newborn Screening in the United States. This wide-ranging study delved into gaps in the current system and provided solutions for the future “The pace of therapeutic discovery is accelerating, a future for which newborn screening must be prepared. The strategies identified in this study offer the first steps toward a modern newborn screening system where all babies in the U.S. have access to a timely diagnosis, thus reducing the risk of preventable injury and death.”
Broad adoption of newborn screening is crucial for our GFPD Community, and we are grateful for continued research and support from the scientific and advocacy communities to make this a reality. Newborn screening programs vary depending on where you live. To learn more about newborn screening and legislation in different US states, please visit The EveryLife Foundation’s Newborn Screening page.

